In a recent article published in the Lancet, researchers performed a multicentre, randomized controlled trial (RCT) at hospitals in ten countries, of which nine were low- and middle-income countries (LMICs) and one was a high-income country, Chile.
The LMICs were Brazil, India, China, Mexico, Pakistan, Nigeria, Peru, VietNam, and Sri Lanka.
Background
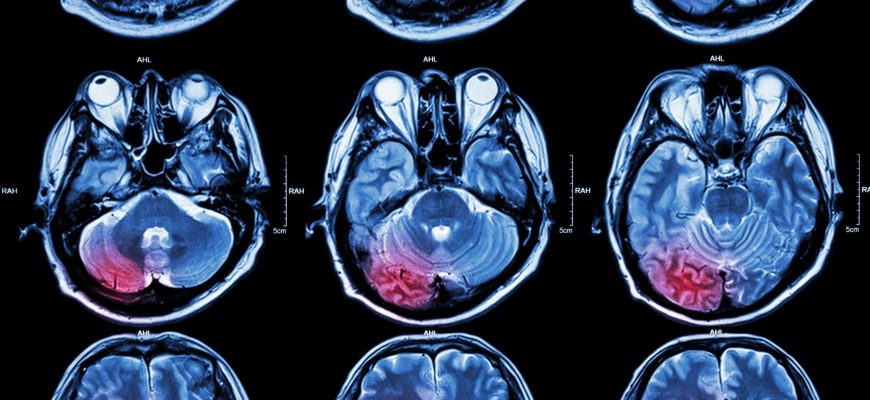
Hypertension, unhealthy diets, e.g., a high salt intake, and many similar risk factors augment the possibility of intracerebral hemorrhage.
Elevated systolic blood pressure (BP) is expected after the onset of intracerebral hemorrhage and is strongly associated with a poor outcome; thus, its early control, i.e., bringing it down to a target of 140 mm Hg or less, appears to be the most promising treatment for acute intracerebral hemorrhage.
Through an intensive screening of published literature, researchers identified three previous trials assessing the effectiveness of a combination of treatments for blood pressure lowering on clinical outcomes of acute intracerebral hemorrhage.
Intracerebral hemorrhage is a type of stroke that is untreatable and accounts for ~20% of the 20 million stroke cases occurring worldwide every year, mostly in LMICs.
None of these trials fetched adequate evidence that implementing an intensive care bundle could effectively combat acute intracerebral hemorrhage.
About the study
In the present study, researchers designed the Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3) to investigate whether implementing a care bundle in a hospital setting could improve outcomes for patients with intracerebral hemorrhage.
This care bundle protocol targeted normalizing the following physiological variables in patients:
i) lowering systolic BP to 140 mm Hg or less;
ii) lowering glucose levels to 6.1–7.8 mmol/L in nondiabetic and 7.8–10 mmol/L in diabetic patients;
iii) treating pyrexia, i.e., maintaining body temperature ≤37.5°C; and
iv) quick reversion of warfarin-associated anticoagulation to attain a normalized ratio of <1.5 in one hour.
The researchers used a modified Rankin Scale (mRS) to measure improvement in functional outcomes six months post-treatment with the care bundle protocol vis-a-vis usual care. Scores between zero to one on the mRS indicate a favorable outcome, i.e., no disability; between two to five indicate increasing disability; and six indicate death.
Time-varying trends in patient characteristics could not explain these results, encompassing favorable effects on health-related quality of life and survival.
All included hospitals followed disease-specific care protocols and were inclined to implement the care bundle for imaging-confirmed spontaneous intracerebral hemorrhage patients aged ≥18.
These patients presented within six hours of symptom onset, had a local guardian, and consented to give the required study data.
The team randomly allocated three implementation sequences, each with four periods, to all participating hospitals, stratified by country and the estimated number of intracerebral hemorrhage patients it would admit during the study period of one year.
The protocol dictated how the hospitals switched from the usual care to care bundle procedure among different patient clusters following a wedged, stepwise fashion.
The trial had a hybrid discovery–implementation design, in which the team prospectively followed up with all patient clusters to establish their outcome.
The team performed all analyses for a modified intention-to-treat population for whom functional outcome data were available.
It helped them assess the distribution in mRS scores, with adjustments for a cluster, e.g., hospital site, time using a proportional ordinal logistic regression model. It presented the standard odds of worse functional outcomes for the care bundle group than the usual care group.
For instance, the team estimated the standard odds of worse neurological deterioration for the care bundle group compared with the usual care group. National Institutes of Health Stroke Scale (NIHSS) scores ranged between zero and 42, where higher scores indicated more severe neurological deficits.
Likewise, combined scores for mobility, self-care, usual activities, pain or discomfort, and anxiety or depression provided an overall health utility score assessed via EuroQoL Group 5-Dimension (EQ-5D-3L) questionnaire.
Results
The modified intention-to-treat population comprised 7,036 acute intracerebral hemorrhage patients from 121 hospitals with a mean age of 62 years; also, 2,533 (36%) were female, and 6,350 (90.3%) were of Chinese origin.
The team allocated 3,221 and 3,815 patients to the care bundle and the usual care groups, respectively. They had primary outcome data for 2,892 and 3,363 patients in the care bundle and usual care groups, respectively.
The care bundle group individuals had better mRS scores than usual care group people, with a common odds ratio (OR) of 0.86 for mean effect across all mRSs violated in the proportional odds assumption test.
In the care bundle group, favorable shifts in mRS scores remained unperturbed even after replacing missing data using prespecified imputation methods, except for an mRS score of six.
These shifts remained significant even after adjustment for patient characteristics, country, and using varying time trends. Furthermore, the care bundle group patients had fewer serious adverse events than the usual care group patients (16% vs. 20·1%).
Conclusions
Previous trials fetched mixed results concerning the effects of blood pressure-lowering therapies in acute intracerebral hemorrhage patients. Perhaps, variable approaches toward treatment and the use of small sample sizes affected the results.
However, in this trial, implementing a simple, targeted care bundle protocol for early intensive management of systolic BP below 140 mm Hg was safe and effectively improved functional outcomes in acute intracerebral hemorrhage patients.
Thus, the authors recommended incorporating it into clinical practice for disease management.